Doctors and fogeys celebrated the key advances that got here in 2023 to deal with respiratory syncytial virus (RSV), which sends as much as 80,000 kids beneath age 5 to the hospital every year within the U.S. This 12 months, the U.S. Meals and Drug Administration permitted two crucial methods to cut back the danger of RSV in younger youngsters: a vaccine for pregnant moms that may defend newborns, and a drug therapy for infants beneath one 12 months.
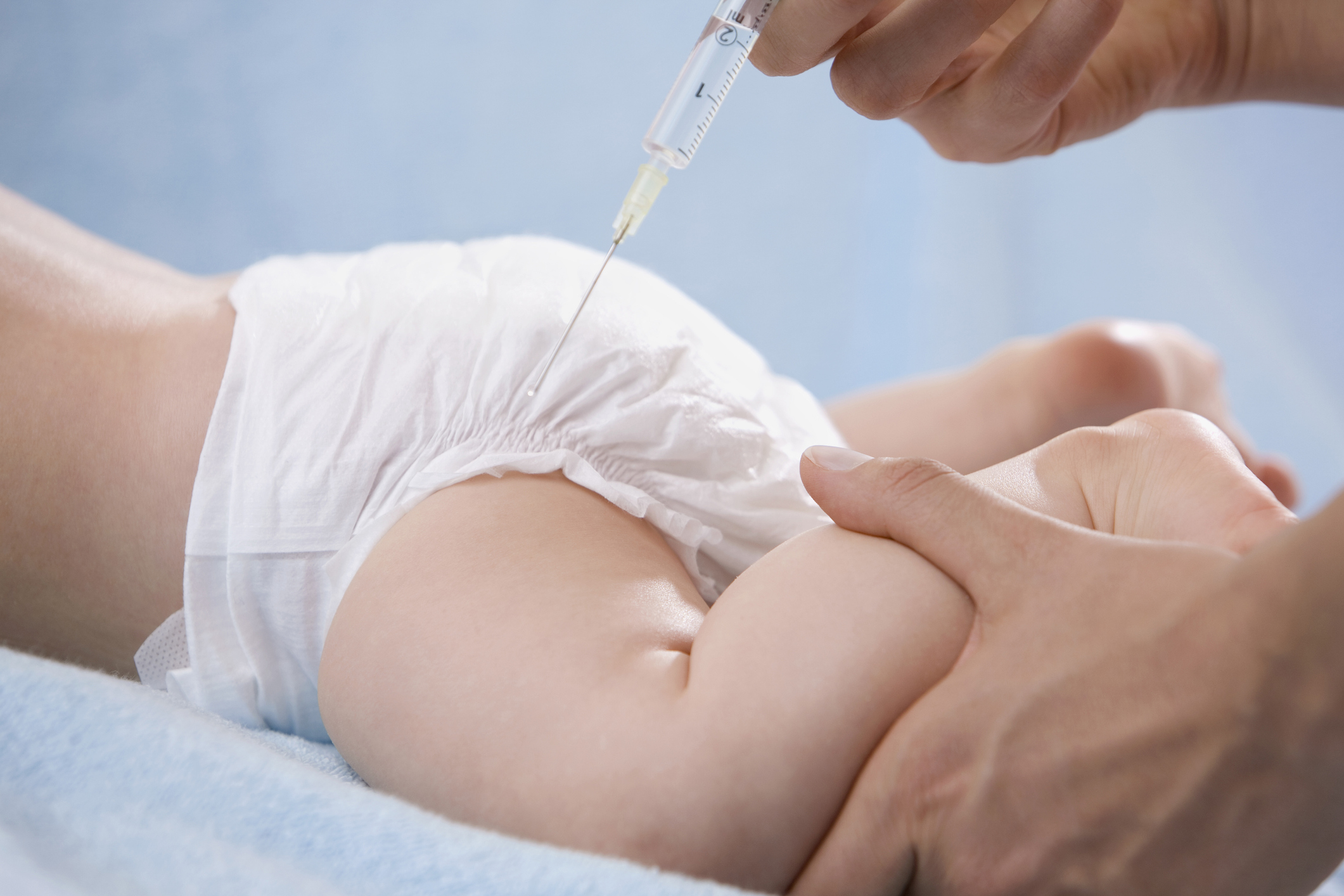
In a examine revealed within the New England Journal of Drugs, researchers report encouraging real-world knowledge that present how efficient the drug therapy, nirsevimab (model title: Beyfortus), could be. The examine, which was funded by the drug’s makers Sanofi and AstraZeneca, included greater than 8,000 infants in France, Germany, and the U.Okay. who had been one 12 months previous or youthful and getting into their first RSV season, which runs from fall to spring. Nivrsevimab is a monoclonal antibody that acts nearly like a vaccine, coaching a baby’s immune system to acknowledge RSV and defend in opposition to it. Half of the infants within the examine had been randomly assigned to obtain nirsevimab, and half obtained no therapy. The drug was 83% efficient in stopping hospitalization amongst these getting it, and 75% efficient in decreasing extreme RSV. These outcomes had been comparable whatever the child’s age, gestational age, or intercourse.
“From a scientific standpoint, it is unbelievable that had been capable of stop greater than 80% of youngsters who obtained the drug from going to the hospital,” says Dr. Saul Faust, pediatrician at College Hospital Southampton and co-leader of the examine.
The findings help suggestions by the U.S. Facilities for Illness Management and Prevention (CDC) that any child beneath Eight months previous obtain a single injection of nirsevimab earlier than their first RSV season if the mom has not already been vaccinated in opposition to RSV. However for the reason that drug was permitted in July, its makers, AstraZeneca and Sanofi, haven’t been capable of sustain with the surge in demand. “The demand for Beyfortus has far surpassed any earlier customary,” an AstraZeneca spokesperson mentioned in response to questions on ongoing points with provide.
In October, the CDC alerted medical doctors about methods to limit provide of nirsevimab to infants at highest threat of RSV problems, similar to youthful and decrease weight infants, and people born with underlying well being situations. The company additionally really helpful that a few of these high-risk infants, together with these born prematurely and who’ve coronary heart and lung situations, proceed to obtain an older, current RSV therapy referred to as palivizumab (model title: Synagis). That may maintain provides of nirsevimab out there for infants who don’t qualify for palivizumab. Whereas paliviziumab is protected and efficient, it requires month-to-month injections all through RSV season, so medical doctors and fogeys had been hoping to begin profiting from nirsevimab’s single-injection.
For households with infants who aren’t at particularly excessive threat of RSV problems, Dr. Rick Malley, a pediatrician at Harvard Medical Faculty and Boston Kids’s Hospital, says there are methods to guard infants even when they can not get the shot. Particularly through the holidays, Malley says mother and father can ask guests to keep away from exposing themselves to the child if they’ve any signs of respiratory infections, similar to a fever, cough, or runny nostril. If guests insist on getting near the child, touching the toddler’s ft reasonably than face may cut back the danger of passing on any infections.
The opposite reassuring information is that basically, RSV infections are inclined to peak earlier within the season, so it is attainable the very best threat is over for this 12 months. Nonetheless, nirsevimab’s producer anticipates that provides will step by step proceed to extend in coming months. “Roughly 230,000 further doses will likely be made out there in mid-January for this RSV season,” says the AstraZeneca spokesperson. “This follows the announcement in November that 77,000 further doses had been being made out there.”