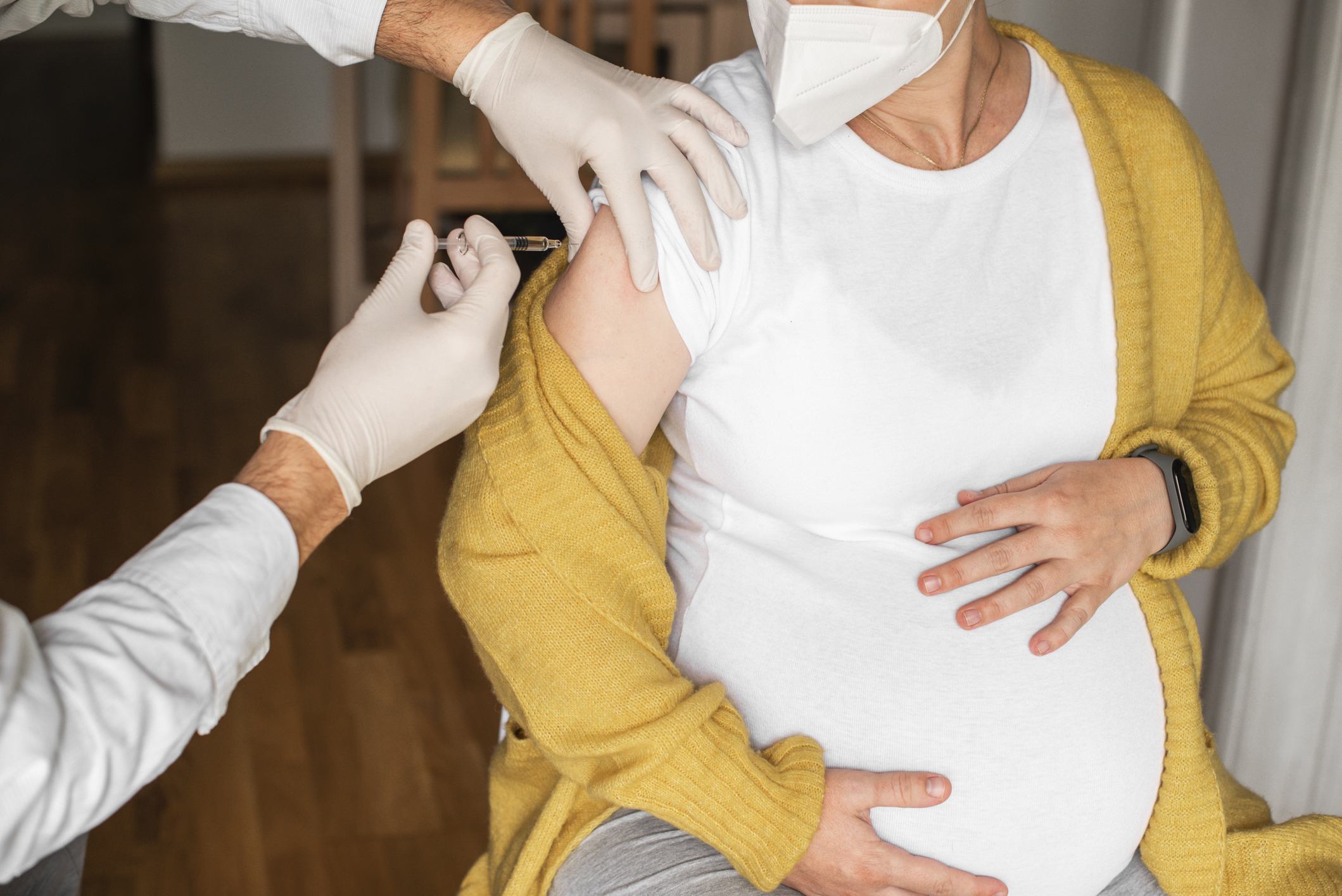
The US Meals and Drug Administration (FDA) immediately (Aug. 21) accredited the primary vaccine for respiratory syncytial virus (RSV) for pregnant ladies, which is designed to guard newborns from the an infection.
The shot turns into the second RSV vaccine, and third new intervention in opposition to the illness this yr. In Might, the FDA accredited the Abrysvo vaccine for stopping RSV in folks 60 years and older. The identical vaccine is now accredited for pregnant folks of their third trimester. And in July, the company accredited nirsevimab, an antibody-based injection that protects infants instantly after start from RSV.
The brand new interventions have been a very long time coming in RSV remedy, which within the U.S. sends 58,000 to 80,000 youngsters below age 5 to the hospital every year and kills 100 to 300 yearly. Pfizer, which makes Abrysvo for each older folks and now those that are pregnant, has been creating the shot for the reason that 2010s, after scientists on the Nationwide Institutes of Well being found simply the fitting type of RSV to seize within the vaccine and current to the immune system. In a research reviewed by the FDA involving pregnant ladies in 18 international locations, the vaccine was 81.8% efficient in stopping extreme respiratory sickness needing medical consideration in infants within the first 90 days after start.
Learn Extra: Why It Took So Lengthy to Lastly Get An RSV Vaccine
Vaccinating moms to guard infants
Stopping an infection is important to defending the well being of new child infants, says Dr. Melissa Squires, a pediatric neuroclinical care physician at Cincinnati Kids’s Hospital who joined the research when she was pregnant together with her second little one in 2019. A part of the rationale she enrolled to change into a participant within the trial was private expertise—when her first daughter, Teresa, was three months previous, she turned very sick with a respiratory an infection and couldn’t breathe nicely or eat or drink correctly. “These have been a really tense few days,” she says. “I don’t know what she had as a result of we didn’t have her examined, however she was fairly sick. Something I can do to stop anybody going via that, I used to be very onboard with exploring.”
Her expertise together with her second daughter has been very completely different. Natalie was born in December 2021, throughout RSV and flu season, so becoming a member of the trial was “an incredible alternative to stop sickness in my child who was going to be born in the midst of respiratory illness season,” says Squires. To date, Natalie hasn’t had a serious an infection via the primary yr of her life. Whereas Squires doesn’t know if she obtained the vaccine or a placebo, she and her husband are blissful to be spared the anxiousness of worrying a couple of sick new child.
Abrysvo works by co-opting the expectant mom’s immune system, prompting it to provide antibodies in opposition to RSV. These antibodies are then handed on via the placenta to the creating fetus, in order that at supply, the newborn has amassed a sure stage of immune defenses that may shield it from any viruses it encounters.
“I’m an enormous believer in maternal vaccination,” says Dr. Elizabeth Schlaudecker, medical director of the division of infectious illnesses at Cincinnati Kids’s and one of many principal investigators of the research. “It’s a really protected and efficient method to shield younger infants when their immune methods aren’t working nicely on their very own fairly but.” Schlaudecker notes there’s precedent to such maternal immunization, together with tetanus, diphtheria and pertussis in addition to flu vaccines, all of which pregnant ladies get through the third trimester to guard their infants after supply. Infants below six months previous are probably the most weak to infections, since their immune methods aren’t developed sufficient but to fend off micro organism and viruses. “Even when we have been to present them vaccines, plenty of them can be unable to mount an immune response as a result of they’re simply too younger,” says Schlaudecker.
Infants are significantly prone to getting severely in poor health from RSV, since their airways are so small, and any irritation brought on by the virus could make it troublesome for them to breathe or clear fluids from their tiny lungs.
Research from Pfizer present that newborns might be protected a minimum of via their first RSV season; research on how lengthy the safety lasts are ongoing.
The upcoming fall and winter season within the northern hemisphere might be a take a look at of kinds for a brand new RSV remedy technique for infants and younger youngsters. With the approval of Abrysvo for pregnant folks, and nirsevamab, which is supposed for newborns of their first RSV season, docs now have efficient methods to stop an infection for the primary time. They should determine the easiest way to make the most of each the maternal vaccine and the antibody remedy for infants to optimize safety from the virus. A few of that steerage will come from the Facilities for Illness Management’s Advisory Committee for Immunization Practices, which is able to element when docs ought to use the brand new vaccine.
Realizing what vaccine to present
“This can be a undoubtedly an unprecedented time,” says Schlaudecker. “There are some distinctive challenges to determining which one to present and when.” She notes the maternal vaccine will match comparatively simply into the present schedule of photographs that expectant moms obtain, and ideally, most pregnant mothers would get the RSV vaccine of their third trimester.
If, nevertheless, the mom isn’t capable of get vaccinated, or if she delivers early and earlier than her scheduled RSV shot, then the antibody remedy could possibly be an essential again as much as shield the newborn. Any infants with weakened immune methods, or extra well being points akin to congenital illnesses that may make them extra weak to infections, may additionally get nirsevimab remedy even when they have already got antibodies from their moms, as a further layer of safety.
The main points of which infants may profit from each having their moms vaccinated, and nirsevimab, will change into clearer as soon as extra information is collected from infants getting these therapies after a yr or two. “I do anticipate that sure, we are going to proceed to do analysis to take a look at how one can incorporate these two merchandise collectively,” says Schlaudecker.
Within the meantime, Squires sees the advantage of benefiting from the maternal vaccine for RSV. “I’m a fan of any type of prevention for RSV,” she says. “Infants can obtain vaccines after the primary yr of life, however even after they do, it takes some time earlier than the physique responds with these antibodies. So let mother do the work. Let mother make these antibodies and provides them to the newborn. That means, on day one they’re able to face that virus.”
Extra Should-Reads From TIME